CDMO Business
Simulated image of Naruto Multi-Active Pharmaceutical Ingredient Plant (NMA)(Operations to be launched in early 2023)
Business Content
- The new facility will be a GMP-conforming multi-purpose plant designed for solid-phase and/or flow synthesis, and capable of handling highly potent substances (OEB Category 4)
- Applying the organic synthesis technologies amassed over long years of manufacturing pharmaceuticals, the plant will provide an integrated service ranging from special raw materials to intermediates and middle-molecular APIs.
- We meet diverse needs with our halogenation technology cultivated over the span of 60+ years and our proprietary catalyst development technology.
| Synthesis in Lab. | GMP production (To be launched in early 2023) |
|
|---|---|---|
| Basic structure | Antisense, siRNA, aptamers, miRNA, etc. | |
| Base | Natural and special (we can also synthesis amidites) | |
| Modifications of phosphorous | PO / PS / chimeras | |
| Indication of manufacturing conditions by customers | Possible | |
| Yield (per batch) | Several mg – several g | Several g – several tens of g |
 Automated oligonucleotide synthesizer
Automated oligonucleotide synthesizer(Cytiva, OligoSynt®)
© 2022 Cytiva - Reproduced by permission of the owner
We provide integrated quality control ranging from raw materials to APIs through processes including analytical method development, related compound separation studies, method validations and stability test.
 LC/MS/MS
LC/MS/MS(Thermo Fischer, Orbitrap Eclipse)
 MALDI-TOF MS
MALDI-TOF MS(Bruker, Autoflex)
| Main Instruments for Analysis | |
|---|---|
| LC/MS/MS (Thermo Fischer, Orbitrap Eclipse) | NMR (Bruker, 500 MHz) |
| MALDI-TOF MS (Bruker, Autoflex) | Toxinometer |
| LC/MS (Waters, BioAccord) | Organic elemental analyzer |
| UPLC (Waters, I-class) | FT-IR (Shimadzu Corporation) |
| UHPLC (Waters, H-class) | Polarimeter (Anton Paar) |
| HPLC (Waters, Alliance) | UV spectrophotometer (Shimadzu Corporation) |
| GC/MS (Shimadzu Corporation) | Ion chromatography (Shimadzu Corporation) |
| HS-GC (Shimadzu Corporation) | TOC analyzer |
Our Strengths
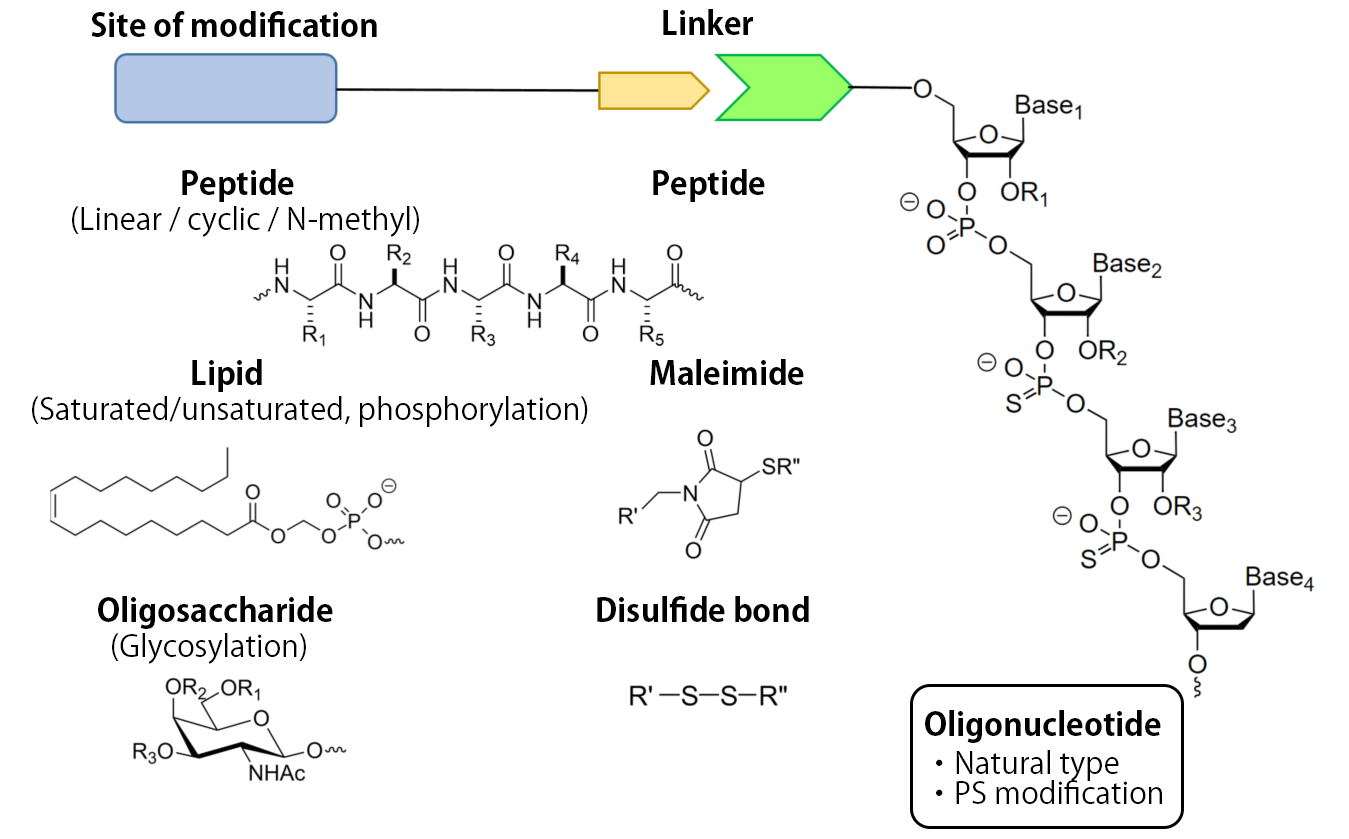
Modified oligonucleotide (by peptides, lipids, and oligosaccharides, etc.)
In addition to oligonucleotide synthesis by conventional solid phase synthesis, we meet the diverse needs of our customers such as modifications through combinations of our synthesis technology plattforms of peptides, lipids and origosaccharides.
Flow Synthesis
What is flow synthesis?
Using a narrow tube as the place of reaction, the flow synthesis method (flow chemistry) is a new chemical synthesis method in which raw chemical reagents continuously mix and react while flowing through a tube.
Compared to the batch synthesis method (a method in which chemical reagents are mixed and react inside a reactor) widely used in industrial manufacturing, the flow synthesis method not only allows flexible adjustment of manufactured quantities, but also makes it possible to implement dangerous reactions that have been difficult to perform in reactor, thus significantly expanding the range and flexibility of the scale of chemical reactions.
In addition, the narrow tube enables fast and precise control of reaction conditions and reagent mixing and, combined with sensing technology, on-demand stable production of various pharmaceuticals, intermediates, raw materials and chemicals can be realized with stability and high purity.
Features of Otsuka Chemical's flow synthesis method
Otsuka Chemical has installed not only flow synthesis equipment capable of handling a wide range of reactions but has also amassed a track record of production using flow synthesis and related know-how through continuous research and development, and can meet diverse customer needs ranging from a mg laboratory scale to medium-volume production of several tens of kg.
Examples of manufacturing and studies
- Hazardous reactions (Cyanation reactions using substances such as KCN, reactions using high-pressure ammonia gas, bromination and iodination reactions using HX)
- Reduction (by H2)
- Halogenation reactions (Bromination using liquid Br2, chlorination using Cl2 gas)
- Organometallic compound synthesis and reactions (synthesis and use of Grignard reagent)
Services
Including setting flow reaction conditions, our flow synthesis experts consistently provide the fastest possible response to needs for g to kg production and purification, including construction of a system optimized for customers’ reaction systems, liquid pumping tests, and monitoring.
 Automatic cleaning and multi-product automatic manufacturing using a switching valve
Automatic cleaning and multi-product automatic manufacturing using a switching valve
 Bromination reaction: Liquid Br2 pumping
Bromination reaction: Liquid Br2 pumping
Halogenation technology including fluorination
Characteristics of Otsuka Chemical’s halogenation technology
Otsuka Chemical has been using Cl2 gas, liquid Br2, HCl gas and HBr liquid on the industrial scale (kg – mt scale) for over 60 years. In addition to chemical synthesis using these general-purpose halogenating agents, we have developed and implemented unique organic synthesis technologies and purification techniques making optimum use of our accumulated halogenation technologies and handling know-how.
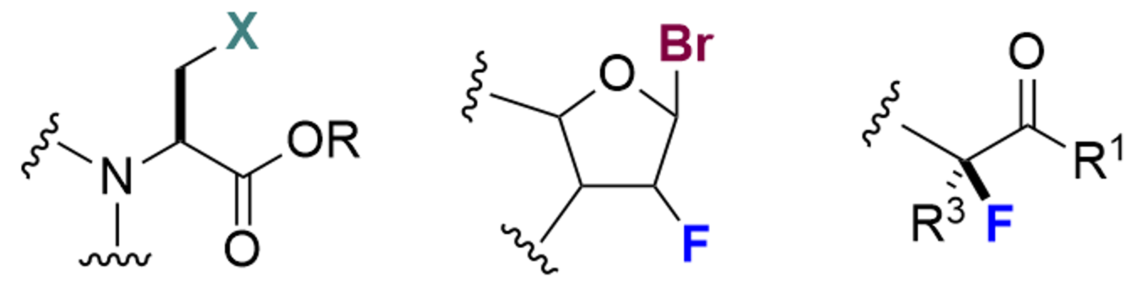
We have also expanded our reactions to include chlorination, bromination, and iodination, and through collaborative research, we are developing a variety of new fluorination reactions, and specialize particularly in the β-position fluorination of carbonyl groups.
Production examples
- Raw materials and small-size molecules for synthesis of middle molecules containing halogens
- Raw materials and small-size molecules for synthesis of middle molecules via halogen intermediates
Services
Including shortening the halogenation reaction route, our experts in handling halogen conduct studies into halogen handling methods and flow combinations and construct a reaction system optimized to consistently meet customer requirements in the shortest possible time.
 Chlorination reaction using chlorine
Chlorination reaction using chlorine
 Ensuring safety by in-house device development
Ensuring safety by in-house device development
Raw materials for synthesis of middle molecules
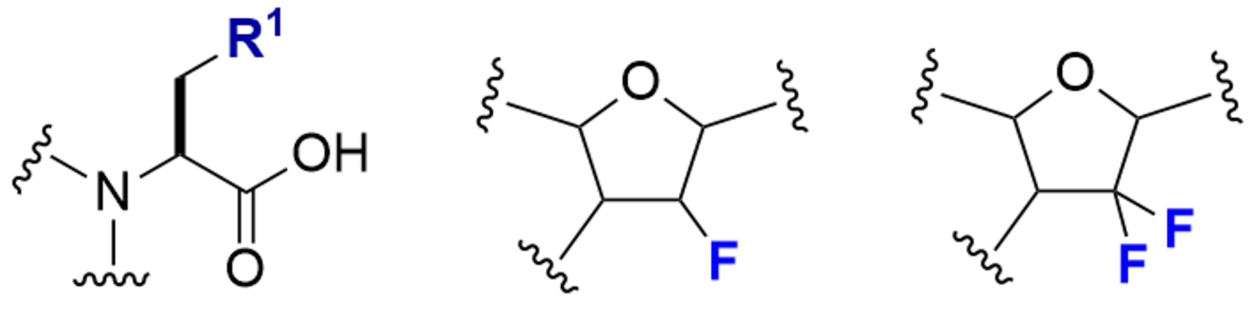
Features
Otsuka Chemical has created new special amino acid and amidite intermediates by combining its halogenation technology with its existing organic synthesis and process technology. The combination of these new intermediates and flow synthesis enables the synthesis of high-quality special amino acids and amidites on the g to kg scale with short delivery times. We have also built a library of as many as hundreds of special amidites and special amino acids in small scale.
We hope you will use our library for drug discovery and library screening.
Click here to see our special amino acid catalog.
 Raw materials for synthesis of special light-emitting middle molecules
Raw materials for synthesis of special light-emitting middle molecules
 Automated library synthesis using robots
Automated library synthesis using robots
Overview of Facilities
Facilities at Naruto Multi-Active Pharmaceutical Ingredient Plant (NMA)
Please feel free to contact us regarding the production of highly potent oligonucleotide drug substance.
| Control level | Equipment | Capacity and specifications | Quantity | Remarks |
|---|---|---|---|---|
| Non-GMP | Solid phase synthesizer | 20 L | 1 | |
| HPLC | 1 | Shimadzu | ||
| GC | 1 | Shimadzu | ||
| Preparative chromatography system | 150 mL/ min | 1 | ||
| 1,000 mL/ min | 1 | |||
| Centrifugal separator | 6 L/ 12000 G | 1 | ||
| Membrane concentrator | 50 L/ h | 1 | ||
| Evaporator | 20 L | 1 | ||
| Freeze dryer | 3 L | 2 | ||
| 20 L | 1 |
| Control level | Equipment | Capacity and specifications | Quantity | Remarks |
|---|---|---|---|---|
| GMP OEB Category 4 |
Solid phase synthesizer | 30 L | 2 | GL |
| 10 L | 1 | GL | ||
| Automated oligonucleotide synthesizer | OligoSynt® (12 mmol) |
1 | Cytiva | |
| High pressure chromatography system | 1.5 L/ min | 1 | YMC | |
| Medium pressure chromatography system | 1 L/ min | 1 | Biotage | |
| Low pressure chromatography system | 0.6 L/ min | 1 | Cytiva | |
| High pressure column | Inner diameter: 100 mm | 1 | Reversed phase large-scale preparative purification system, SUS | |
| Inner diameter: 150 mm | 1 | |||
| Reactor | 30 L | 1 | GL | |
| Evaporator | 30 L | 2 | GL | |
| Membrane concentrator | 30 L/ h | 1 | Cytiva | |
| Freeze dryer | Condenser Capacity: 35 L |
1 | Shelf type | |
| Purified water production system | 300 L/ h (25℃) | 1 | RO + UF membrane + Ion exchange |
 Preparative HPLC column
Preparative HPLC column(YMC, 150mm, 100mm)
Concept image of completed Naruto Multi-Active Pharmaceutical Ingredient Plant (NMA)

QA/ QC
Otsuka Chemical launched its API business in 1992.
We comply with pharmaceutical regulations not only in Japan but also globally, including DMF and ASMF registration and conformity certification.

Tokushima Laboratory
Work such as development of synthetic processes and study on related compounds is handled by our highly-experienced and dedicated staff.
For Oligonucleotide synthesis, we have an OligoPilot ® in our Tokushima laboratory, which enables us to provide gram-scale samples.
Shonan Laboratory
With “open innovation” and “building a next-generation research environment” as the keywords, the Shonan Laboratory is continuously searching for seeds and creating core technologies with partners.
We are capable of undertaking a full range of services from synthesis of various small-size molecules including special amino acids and amidites to peptide and oligonucleotide synthesis.
Click here to see our specialty amino acid catalog

Location
Contact
Life Science Division, Chemicals Business Headquarters, Otsuka Chemical Co., Ltd.
https://www.otsukac.co.jp/contact/
Please feel free to contact us.